
ADCC = antibody-dependent cellular cytotoxicity; ADCP = antibody-dependent cellular phagocytosis; ADCR = antibody-dependent cytokine release; ADCT = antibody-dependent cellular trogocytosis; AKT = protein kinase B; BBB = blood-brain barrier; CNS = central nervous system; EGF = epidermal growth factor; EGFR = epidermal growth factor receptor; ERK = extracellular signal-related kinase; HGF = hepatocyte growth factor; HGFR = hepatocyte growth factor receptor; MAPK = mitogen-activated protein kinase; MOA = mechanism of action; NK = natural killer; NSCLC = non-small cell lung cancer; PI3K = phosphatidylinositol 3 kinase; TKI = tyrosine kinase inhibitor.
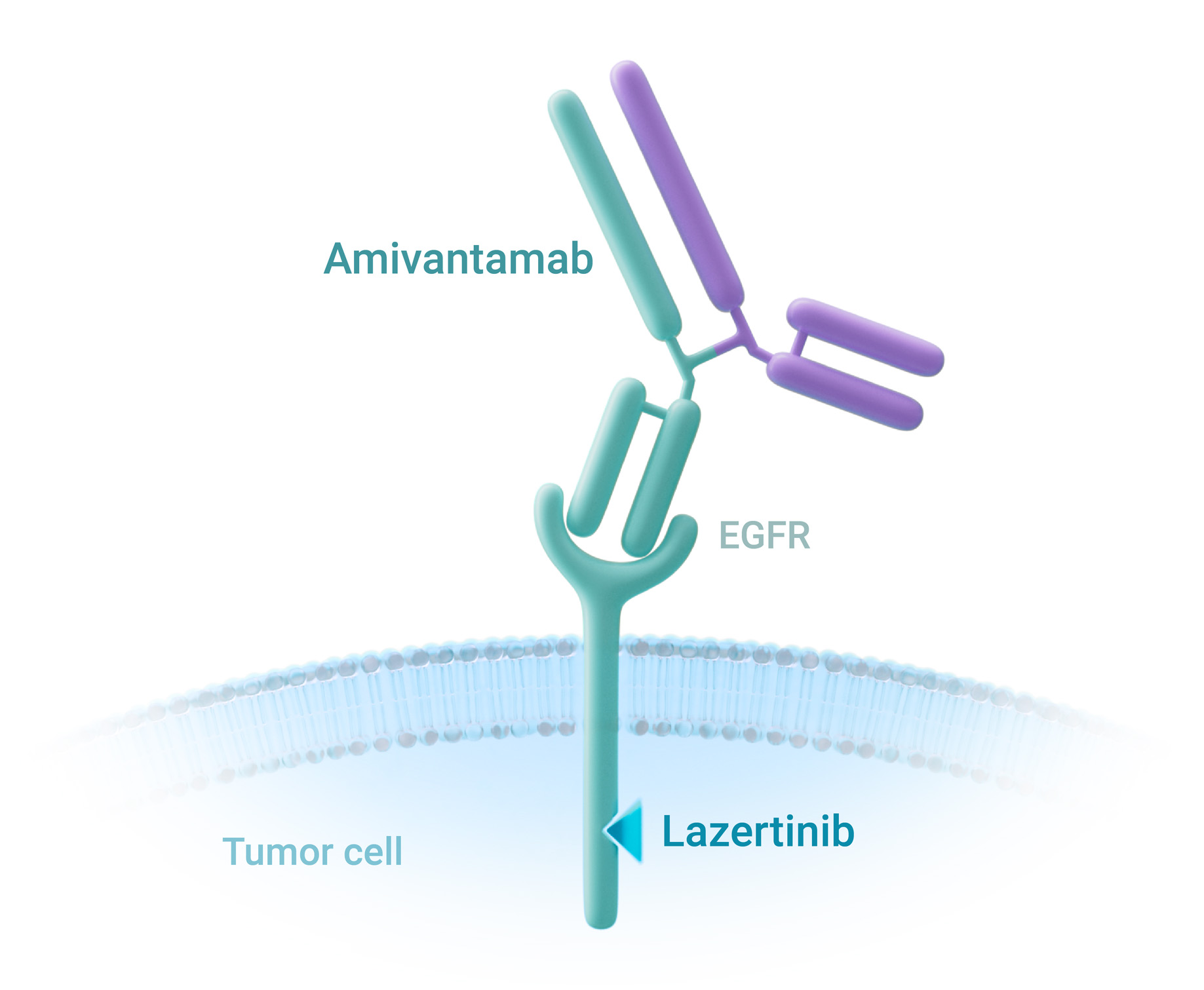
Patients with EGFR -mutated NSCLC are often treated with EGFR TKIs, although some more uncommon mutations are resistant to TKI therapy.4,5
Amivantamab is a
first-in-class bispecific antibody targeting both the EGFR and MET
signaling pathways by binding the extracellular regions of the
respective receptors.3,4
Amivantamab reduces ligand-induced receptor activation by blocking the binding of EGF to EGFR, and HGF to MET.4,13
The binding of the Fc region of amivantamab to the Fcγ receptors on immune cells induces several effector functions through activation of the immune cells. Of these, trogocytosis is the most prominent activity.4,15
Amivantamab is a bispecific antibody that acts via three different mechanisms of action and has a design tailored around higher selectivity and specificity.3,4
The EGFR gene is a key oncogene that is mutated in different cancer types, including NSCLC, and regulates cell proliferation and survival.1
A majority of NSCLC tumors eventually acquire resistance to therapy through various mechanisms, leading to disease progression.4,10–12
Amivantamab inhibits receptor activation and downstream signaling through three separate mechanisms of action (MOA).4
Amivantamab reduces downstream signaling as a result of EGFR and MET receptor degradation following receptor internalization.4,13
Other immune cell-directed activities include cellular cytotoxicity, phagocytosis, and cytokine release.4,14